While using the balls and sticks display, every atom in the protein is visible as well as the chemical bonds between the atoms, but it is hard to determine what secondary structures are present in the protein. We will return to the Jmol workspace and change the protein display to secondary structure display. Right click the mouse to open the additional options menu. In the options menu pick
Style ➔ Scheme ➔ Cartoon
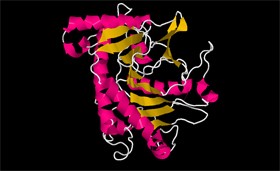
- Specify which secondary structures exist in the IPNS protein
- Describe how the structures are represented in the secondary structure display
There are three main types of secondary structures in the protein, and they are:
Alpha helix – as its name would suggest – is a helical structure like a spring (Figure 3). The spiral shape is formed by hydrogen bonds (white dotted line) binding each amino acid to the amino acid located four spaces further along the protein sequence.
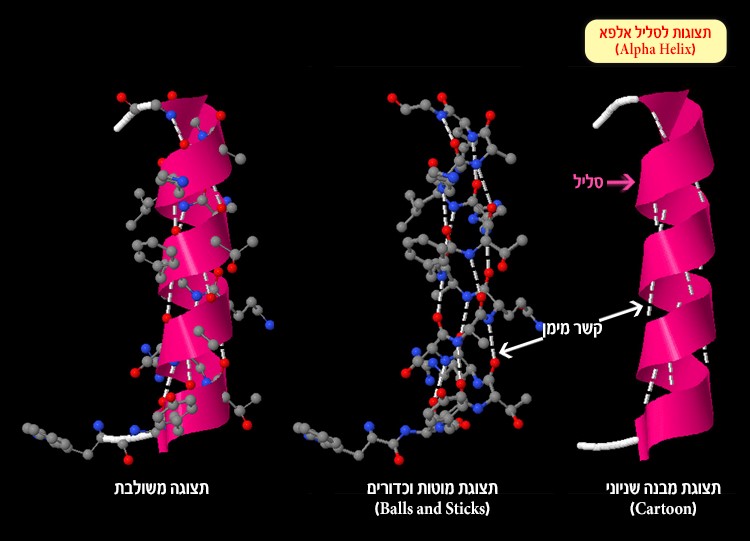
Figure 3: Alpha helix and hydrogen bonds (white dotted line) created between the oxygen atom or nitrogen atom and hydrogen atoms
Beta sheet – a sheet-like structure formed by hydrogen bonds between parallel segments of the polypeptide chain (Figure 4). Each segment in the beta sheet is called a strand.
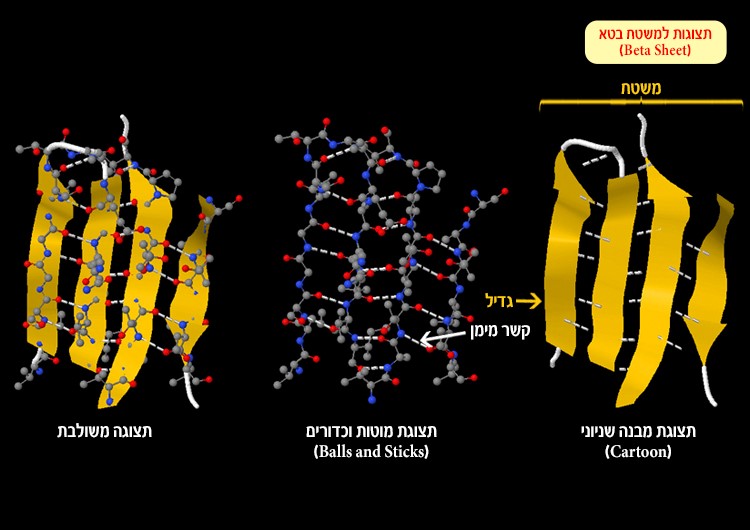
Figure 4: Beta sheet and hydrogen bonds (white dotted line) formed between an oxygen atom or nitrogen atom and hydrogen atoms
Turn and loop – these structures change the direction of the polypeptide chain (the protein chain). They are connective regions between alpha helices and beta sheets (Figure 5).
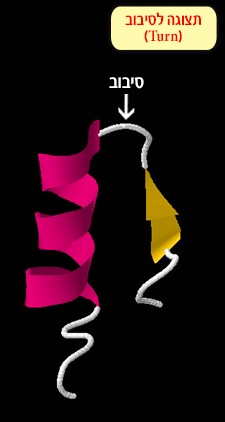
Figure 5: A loop connecting between an alpha helix and beta sheet
Now we return to the IPNS protein.
- Two beta sheets are visible in the enzyme structure; there are 3 strands in one sheet (screen 5). How many strands are there in the second sheet?
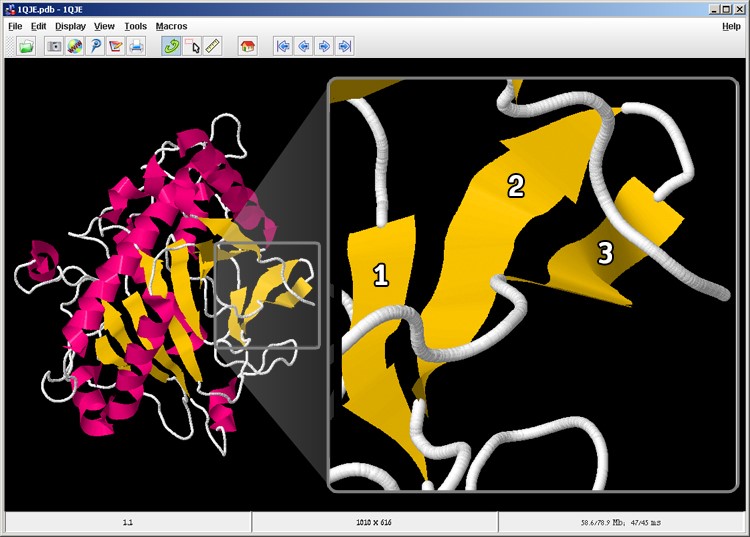
Screen 5: there are two beta sheets in the IPNS enzyme. On the right you can see a magnification of the beta sheet marked with a grey square, while each beta strand is numbered.
Choose one answer:
- One strand
- Three strands
- Eight strands
- It is impossible to know
The correct answer is: C. As seen in the figure, the second beta sheet is composed of eight strands. For your convenience, each beta strand in the sheet is numbered.
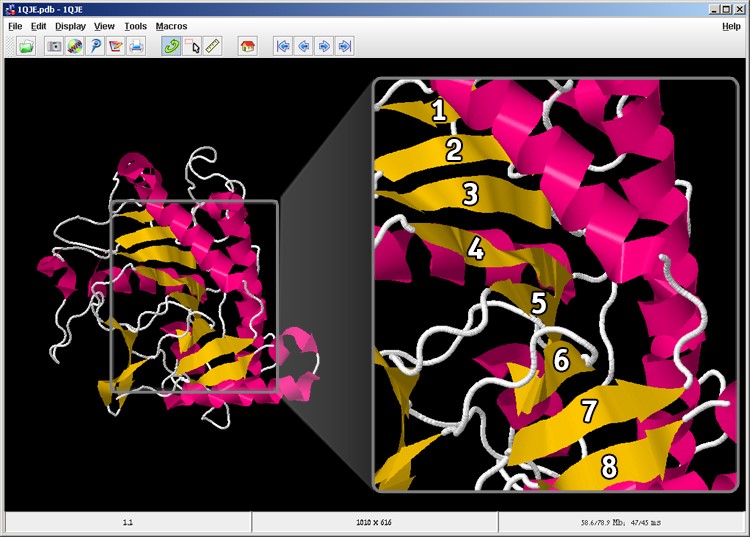 Figure 6: There are eight strands in the second beta sheet in the protein.
Figure 6: There are eight strands in the second beta sheet in the protein.