A search through a database of motifs and protein families using the tool Prosite, discovered that the CFTR protein is an ion channel that belongs to a protein family called ABC (ATP-Binding Cassette). Proteins of this family have two types of conserved motifs: one that anchors the protein to the cell membrane (ABC_TM1F) and another that binds ATP that is required for the active ion transfer against the concentration gradient (ABC_TRANSPORTER2) (Figure 3).
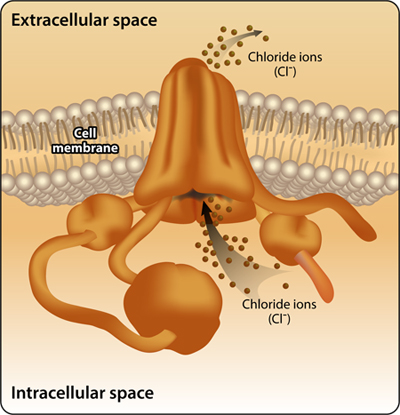
Figure 3: The CFTR protein has four motifs of two types, both essential for its function as an ion channel in the cell membrane: a motif anchored to the cell membrane (named ABC_TM1F) that forms a channel structure for the ion efflux through the cell membrane, and a motif that binds and hydrolyzes ATP (named ABC_TRANSPORTER_2) to support an active ion transfer against the concentration gradient.
At the beginning of the protein, the N terminal site, lies the cell membrane anchoring motif, followed by the ATP binding motif and then by another anchoring motif. Last, at the C terminal end lies another ATP binding motif. The motifs are independent one on the other and are structurally separated, yet they “cooperate” together. The cell membrane anchoring motifs form a channel structure for ion transfer. The energy released from ATP dissociation by the ATP- binding motif, allows the active transport of chloride ions through the cell membrane against the concentration gradient. Based on the identified motifs and their positions, we now drew a model that explains how CFTR functions as a chloride ion transporter. Prosite played a key role by identifying motifs that are essential for CFTR function and supplying information on their individual roles; we learned about the relative positions of these motifs and recognized the amino acids involved in activities such as ATP binding and dissociation. To summarize, through Prosite we identified motifs in the CFTR protein and learned about their significance to the structure and function of the protein as an ion transfer channel in the cell membrane.